What is the evidence supporting varenicline plus nicotine patch versus varenicline alone for smoking cessation?
Introduction
Introduction
The reduction in tobacco smoking in the United States (US) over the past 50 years is one of the most remarkable public health achievements in modern history.1 The prevalence of cigarette smoking among US adults in 2018 was almost 14%, the lowest ever recorded. The introduction and advancement of treatment options for smoking cessation are partly responsible for this reduction in tobacco use.
The gold standard for smoking cessation treatment is a multimodal approach combining behavioral and pharmacologic therapies.1-3 Various pharmacologic agents with different mechanisms of action are available. Choice of pharmacotherapeutic medication is dependent on multiple factors such as patient preference and comorbid conditions. Additionally, the use of combination nicotine replacement and varenicline as part of a multimodal strategy has been evaluated and discussed in treatment guidelines.
Literature review
Literature review
The combined use of varenicline plus the nicotine patch is recommended as first-line therapy for smoking cessation by the American Thoracic Society (ATS) guideline (2020), but not by an American College of Cardiology expert consensus statement (2018) or the Surgeon General (2020).1,2,4 The ATS recommendation is “conditional, [with] low certainty in the estimated effects.” This lack of consensus among the guidelines may be attributed to studies that indicate inconsistent efficacy of combination therapy.
A 2009 retrospective study by Ebbert et al. first investigated the safety and efficacy of combination treatment with varenicline and nicotine replacement therapy (NRT).5 The analysis involved 2 cohorts of patients enrolled in a residential tobacco treatment program: patients receiving combination treatment with varenicline and NRT and patients receiving treatment with NRT only (“usual-care” patients). In the combination treatment group, 71% of patients used the nicotine patch and added a short-acting NRT (inhaler, lozenge, nasal spray, and gum). The 30-day point prevalence smoking rate at 6 months was 54% (95% confidence interval [CI], 44% to 64%) in the combination treatment group, compared to 59% (95% CI, 50% to 66%) for usual-care patients. Adverse events were experienced by 39% (95% CI, 31% to 49%) of patients in the combination treatment group and by 59% (95% CI, 51% to 67%) of usual-care patients. The investigators concluded that combination therapy with varenicline and NRT was safe and tolerable, but not superior to monotherapy. A potential limitation to this study is that the 2 cohorts were from different time periods (cohort 1: June 2006 through September 2008 and cohort 2: January 2004 through June 2006). Changes in management of smoking cessation over time were not accounted for and could have affected the results.
Three randomized, controlled trials (RCTs) assessing safety and efficacy subsequently compared varenicline plus nicotine patch versus varenicline alone for smoking cessation.6-9 Patients included in the RCTs were at least 18 years of age, not pregnant or breastfeeding, and had no psychiatric illness. Koegelenberg et al. demonstrated that combination therapy was more effective than varenicline alone for achieving continuous abstinence at 12 and 24 weeks, while the other 2 RCTs did not show superior effects of combination therapy. Table 1 summarizes the number of participants, interventions, outcomes, and results of the RCTs.
Table 1. Summary of randomized controlled trials comparing combination therapy to monotherapy for smoking cessation.7-9
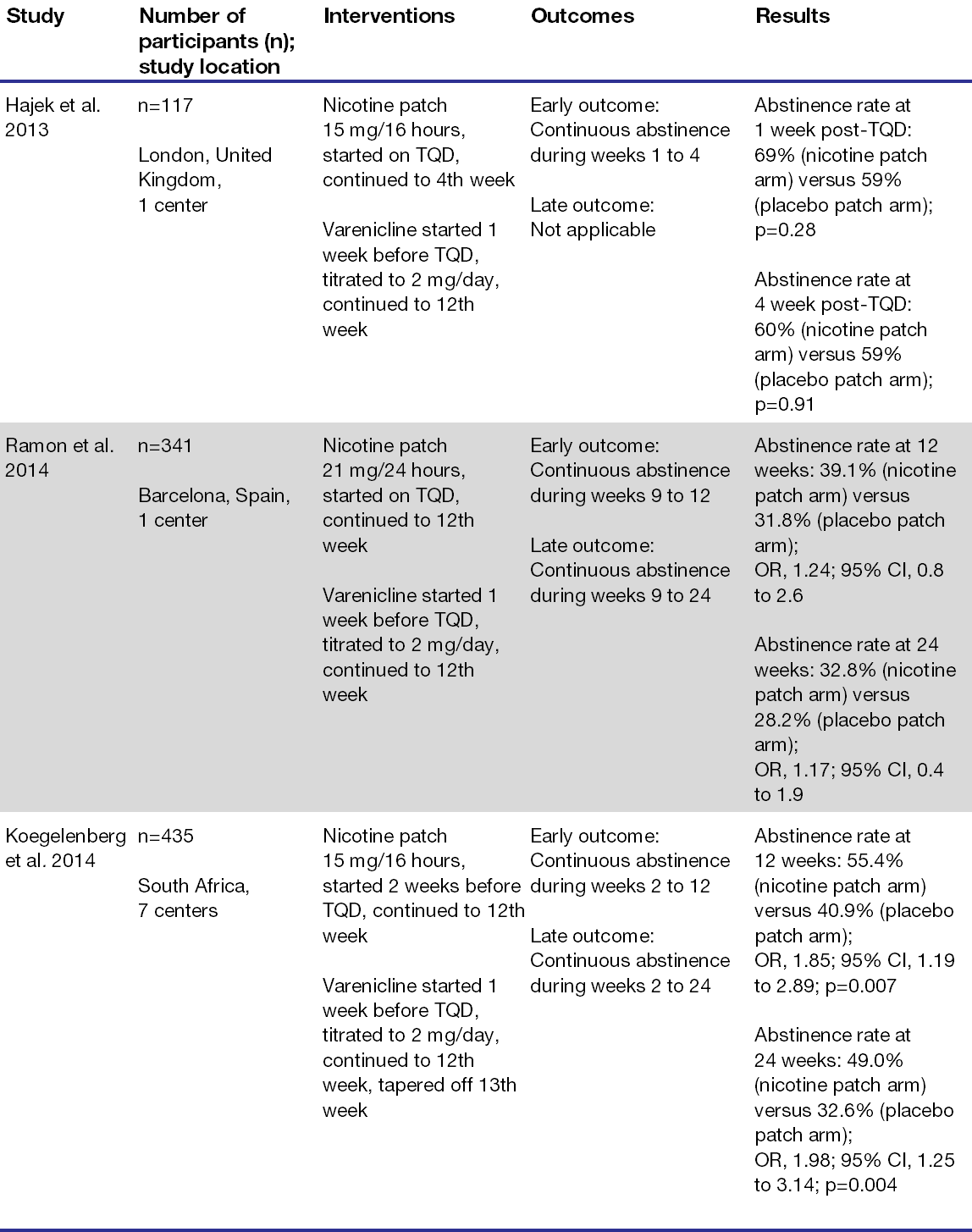
Abbreviations: CI=confidence interval; OR=odds ratio; TQD=target quit date.
These 3 RCTs were also included in a systematic review and meta-analysis that investigated the safety and efficacy of varenicline combined with NRTs.6 The early and late outcomes of continuous abstinence for each RCT are defined in Table 2. The largest RCT (Koegelenberg et al.) significantly impacted the results within a sensitivity analysis, but once eliminated, the favorable effects of the early and late outcomes became insignificant. The systematic review also pooled safety data from the RCTs. The most common adverse events were nausea, insomnia, abnormal dreams, and headache. Except for skin reactions reported in Koegelenberg et al., the adverse effects from combination therapy were comparable to varenicline monotherapy. The study authors concluded that combination therapy with varenicline and the nicotine patch is more effective than varenicline monotherapy and both have similar safety profiles.
Table 2. Meta-analysis of continuous abstinence outcomes from randomized controlled trials.6
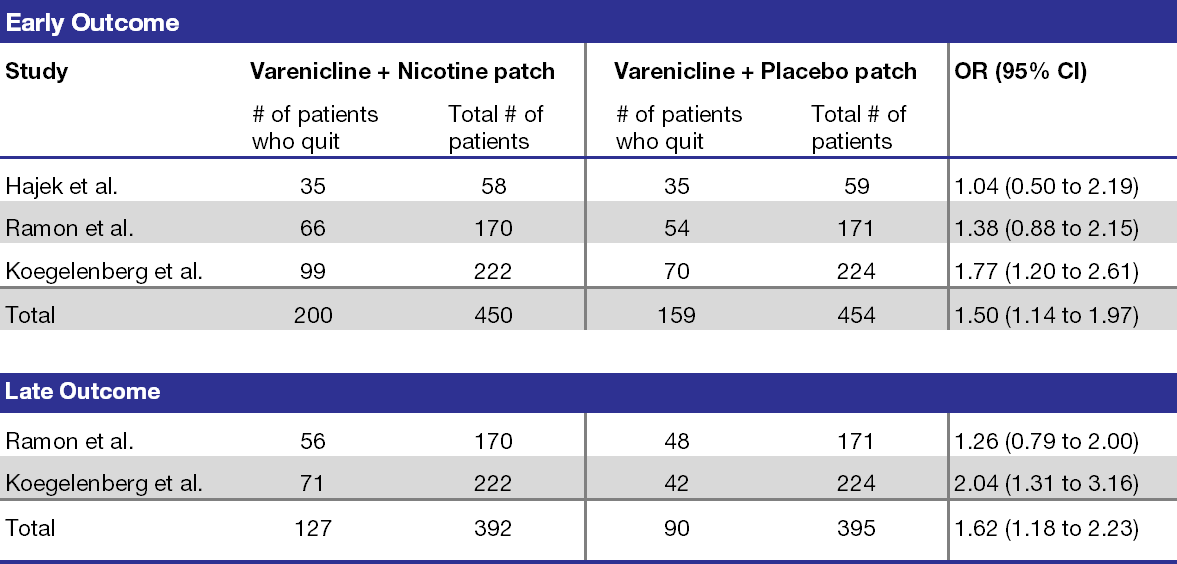
Abbreviations: CI=confidence interval; OR=odds ratio.
Conclusion
Conclusion
Despite being recommended in the ATS guideline, there is limited evidence to support the use of varenicline plus nicotine patch versus varenicline alone for smoking cessation. The only available systematic review concluded that combination therapy is more effective than varenicline monotherapy primarily based on the results of one trial (Koegelenberg et al.), which limits the strength of the evidence.6 Since the publication of the systematic review, newer studies have not further investigated the efficacy and safety of combination therapy with varenicline and the nicotine patch. Larger RCTs are warranted to make more robust conclusions.
References
References
- U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020.
- Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
- Fiore MC, Jaen CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158-176. doi:10.1016/j.amepre.2008.04.009
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11(5):572-576. doi:10.1093/ntr/ntp042
- Chang PH, Chiang CH, Ho WC, Wu PZ, Tsai JS, Guo FR. Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: a systematic review and meta-analysis of randomized controlled trials. BMC Public Health. 2015;15:689. doi:10.1186/s12889-015-2055-0
- Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med. 2013;11:140. doi:10.1186/1741-7015-11-140
- Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med. 2014;12:172. doi:10.1186/s12916-014-0172-8
- Koegelenberg CF, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155-161. doi:10.1001/jama.2014.7195
Posted on Dec. 6, 2022
Last updated on Dec. 6, 2022
Category: Smoking Cessation
Prepared by:
Honey Joseph, PharmD
PGY2 Drug Information Resident
University of Illinois Chicago College of Pharmacy
The information presented is current as of June 2022. This information is intended as an educational piece and should not be used as the sole source for clinical decision-making.